Exploring the molecular basis of host-pathogen interactions
Our lab is in interested in understanding how membrane protein complexes mediate interactions at the host-pathogen interface in endogenous malaria parasites, using biochemistry, parasite genetics, and the latest advances in single-particle cryo electron microscopy and in situ cryo electron tomography.
Integrated Structural Parasitology of Malaria Parasites
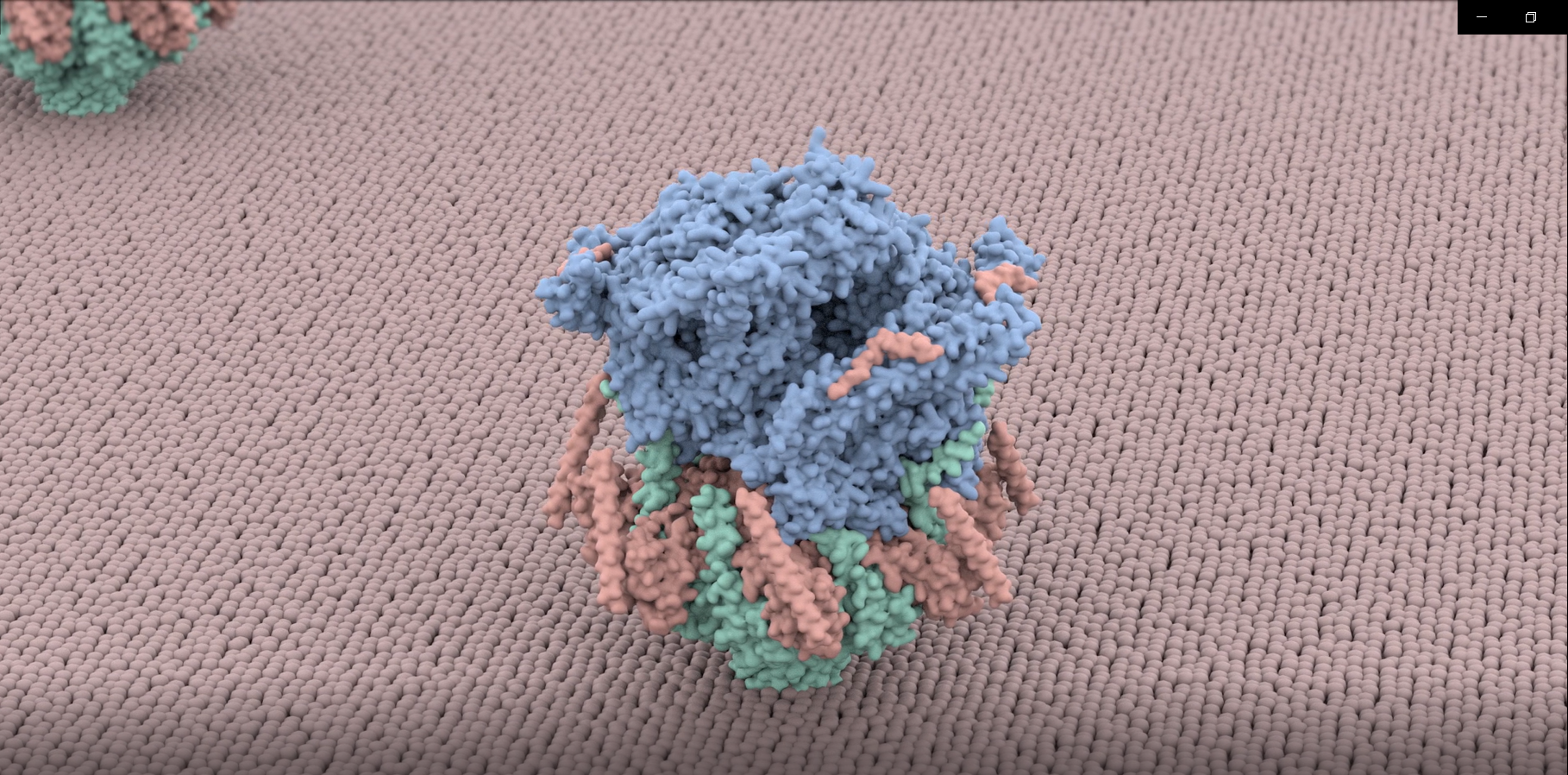
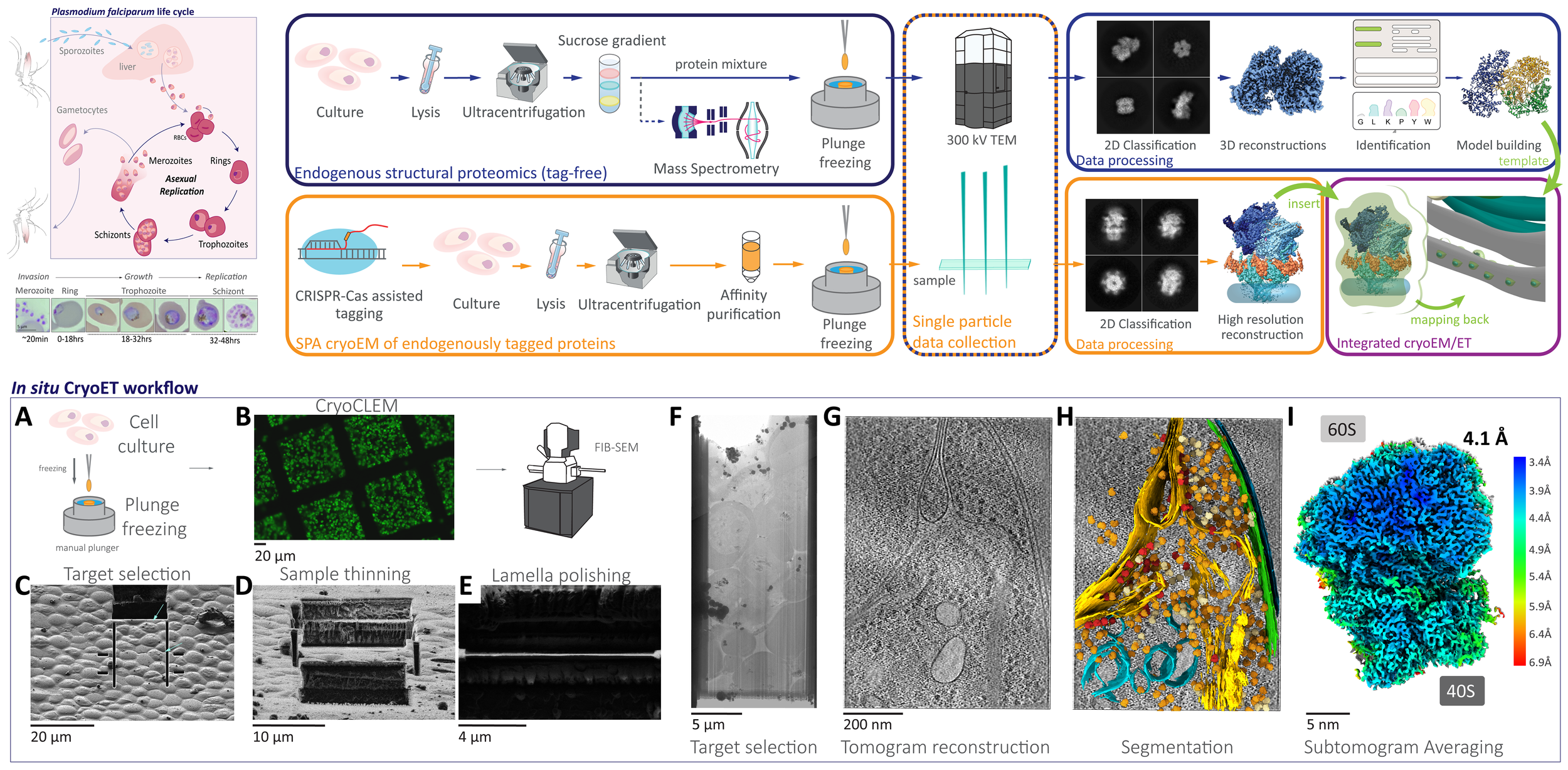
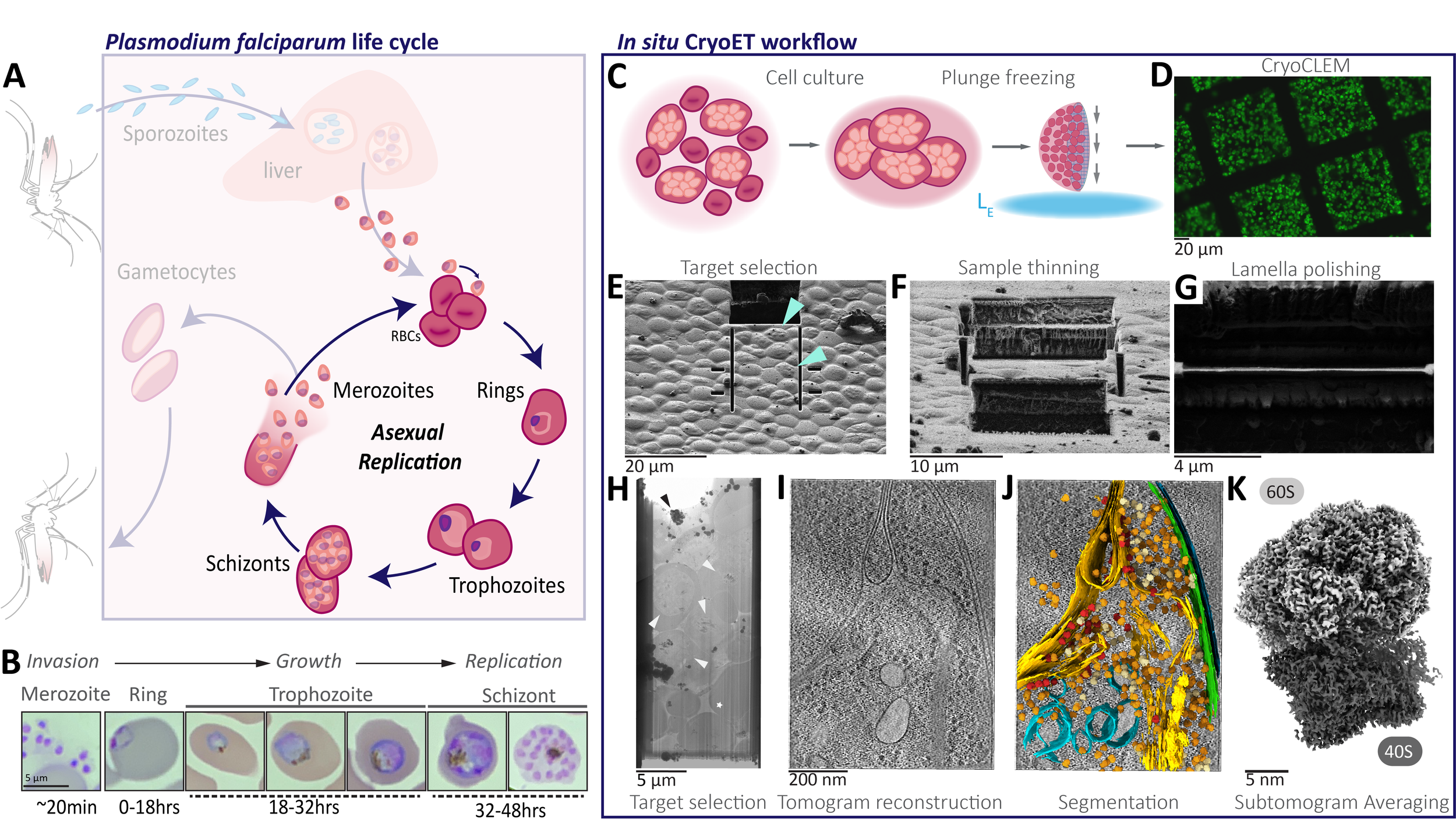
The complexity and breadth of its host-cell remodeling machinery make P. falciparum a rich and exciting system for the study of host-pathogen interfaces. However, many of the molecular mechanisms underlying this parasite’s ability to hijack human red blood cells remain enigmatic, as much of the P. falciparum proteome has proven recalcitrant to structural and biochemical characterization using traditional recombinant approaches. Our lab uses single-particle cryo electron microscopy to determine near-atomic resolution structures of previously intractable protein complexes enriched directly from endogenous P. falciparum parasites, and cryoFIB-enabled in situ cryo electron tomography to directly visualize proteins at the host-pathogen interface in parasite-infected red blood cells at sub-nanometer resolutions.
While most intracellular pathogens export a limited repertoire of effector proteins to co-opt existing host-cell metabolic machineries, the malaria-causing parasite Plasmodium falciparum exports more than 10% of its proteome into its host, the human red blood cell, which the parasite inhabits and reproduces within during the blood stages of its life cycle. The hundreds of proteins in the P. falciparum exportome extensively remodel host erythrocytes, creating the infrastructure needed to import nutrients, export waste, and evade the host immune system. The export of these hundreds of proteins is complicated by the fact that the malaria parasite conceals itself inside a parasitophorous vacuole (PV) derived from invagination of the host cell plasma membrane during invasion. Following secretion into the PV, proteins destined for export must be unfolded and transported across the PV membrane (PVM) into the host cell in an ATP-dependent process.
Mechanism of effector protein export in P. falciparum
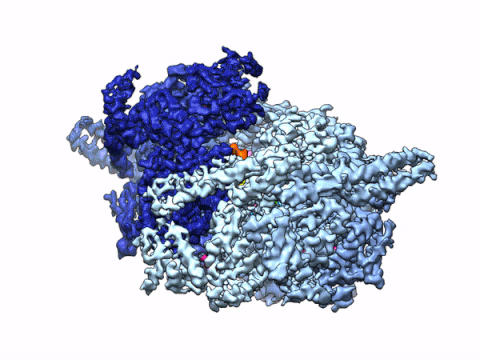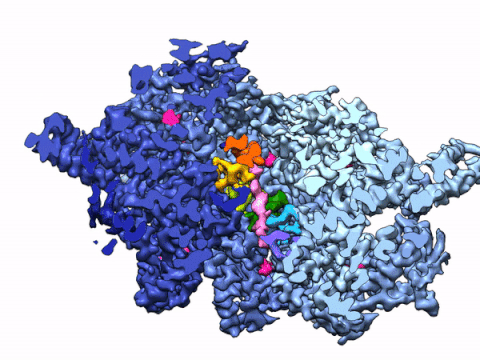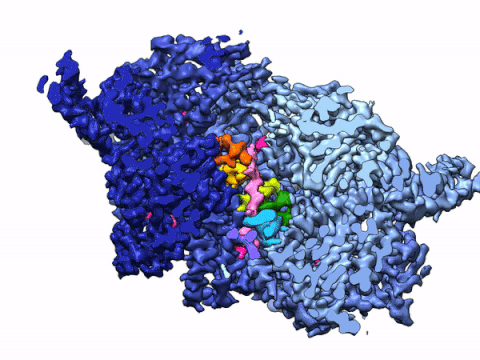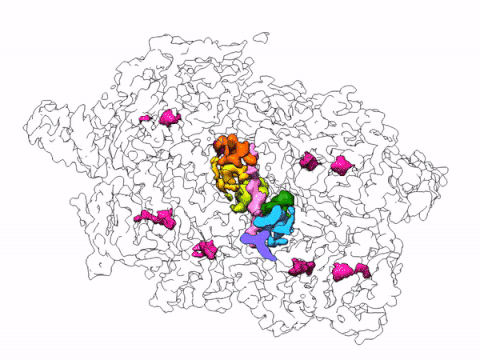
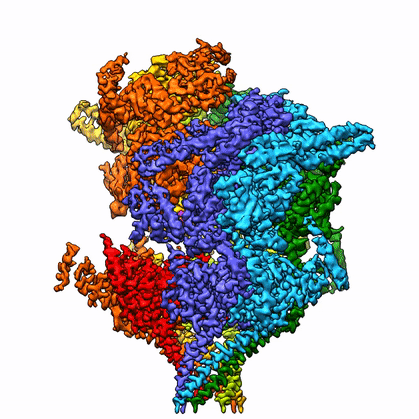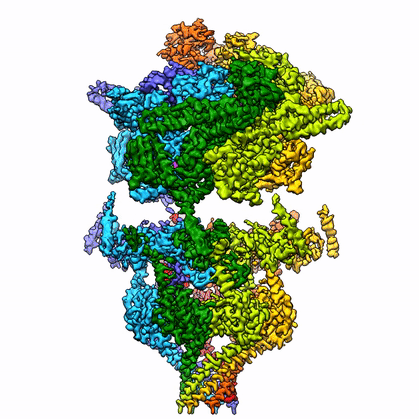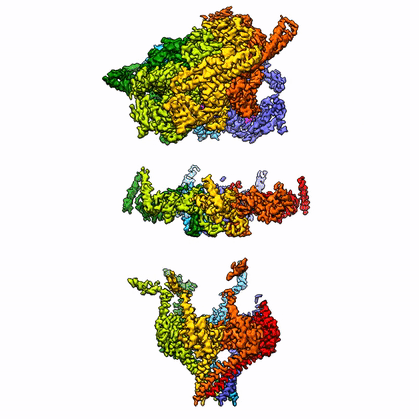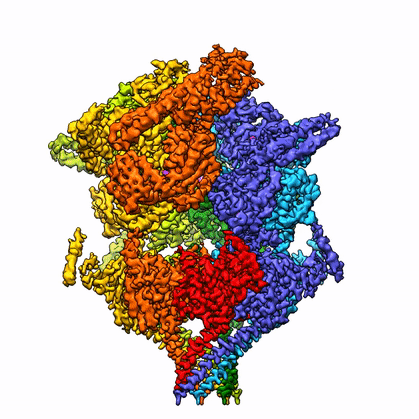
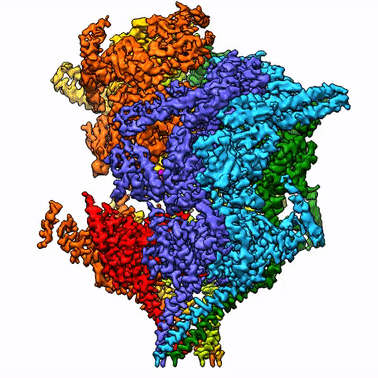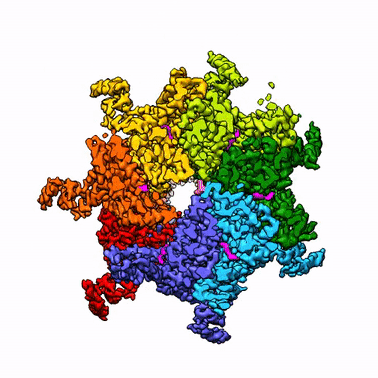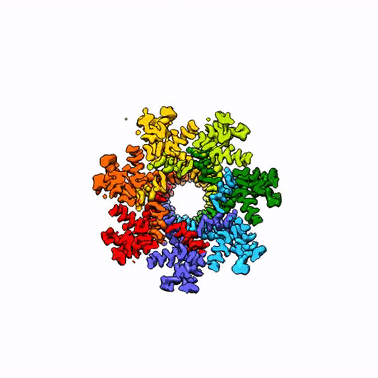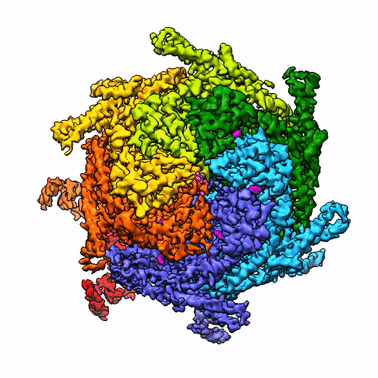
By leveraging the latest innovations in cryo-electron microscopy (cryoEM) and CRISPR-gene editing, we recently determined near-atomic resolution structures of a unique malarial translocon, purified directly from P. falciparum parasites in multiple functional states. Known as the Plasmodium Translocon of Exported Proteins (PTEX), this novel membrane protein complex is the only known point of entry to the host cell for exported proteins and an attractive drug target, as disrupting PTEX blocks delivery of key virulence determinants, inducing parasite death.
Our structures of PTEX are the first near-atomic resolution cryoEM structures of a protein isolated directly from an endogenous source using an epitope tag inserted into the endogenous locus with CRISPR-Cas9 gene editing. This approach is broadly applicable to structural studies of protein complexes from many organisms that have thus far eluded structural characterization using conventional recombinant approaches.
With the ability to obtain near-atomic resolution structures from samples enriched directly from endogenous sources, many previously intractable protein complexes are now within our reach. Ongoing work in our lab is focused on using this approach for further structural and functional characterization of PTEX accessory proteins and their interactions with the PTEX core complex.
Endogenous structural Proteomics
Identifying and characterizing novel protein complexes that mediate host-pathogen interactions is crucial for understanding the molecular mechanisms underlying pathogenesis. Unfortunately, many pathogens of high medical relevance have resisted structural characterization using traditional recombinant approaches. This is particularly so for P. falciparum, where the paucity of high resolution structural and functional information is compounded by the fact that 50% of the P. falciparum proteome is novel. To address the scarcity of structures from P. falciparum and other challenging pathogens, we are developing and implementing methodologies for structure determination from endogenous sources. Initial efforts have yielded multiple near-atomic resolution structures of protein complexes enriched directly from malaria parasites.
In situ imaging of host-pathogen interfaces at sub-nanometer resolution
The ultimate goal in structural biology is to directly visualize protein complexes in action in living cells, at near-atomic resolution. The recent and ongoing resolution revolution in cryoEM is bringing us increasingly closer to attaining this goal. We use cryo focused ion beam-scanning electron microscopes (cryoFIB-SEM) to create extremely thin sections of intact cells called lamella, which are ideal for cryo electron tomography. Using this approach, we directly visualize the host-parasite interface in P. falciparum parasites at high resolution.